CASE STUDY
Decentralized Excellence:
People Science and Phylos Bioscience Show that THCV Improves Energy and Wellbeing with Minimal Side Effects
Study Overview
A look at the study facts, key findings, and real-world implications.
Study Snapshot
Quick facts on how the study was designed, built and run.
Primary focus
Anxiety, sleep
Participants
210 participants, 53% women
Study Design
exploratory, double blind, randomized
Criteria
exploratory, double blind, randomized
Duration
2 weeks recruitment, 1 week baseline, 12 weeks intervention
Data Collection
Oura ring, GAD-7 questionnaire
Validation
IRB approved, published in Beneficial Microbes
Key Results
A look at the most meaningful results, including outcomes and study quality.
20%
Enhanced Energy and Well-Being
40%
Reduced Fatigue
15%
Stable appetite
99%
Compliance
99%
Completion
Study Details
Introduction
The cannabinoid research landscape is rapidly advancing. THC and CBD have long dominated the field, but attention is shifting toward rare cannabinoids with distinct physiological effects, tetrahydrocannabivarin (THCV).
Tetrahydrocannabivarin (THCV), a non-psychoactive variant of tetrahydrocannabinol (THC), presents a promising wellness alternative to THC-only products and traditional medicines. However, clinical evidence is limited, as are descriptions of real-world differences from impacts of THCV. Though present only in trace amounts in traditional cannabis strains, THCV and other rare cannabinoids show promise in the treatment of inflammation, anxiety and metabolic disorders.
Phylos Bioscience, a leader in cannabis genetics, embarked on an innovative journey to validate the efficacy of its THCV+THC gummy, purported to improve energy and sharpen focus while minimizing the fatigue and hunger associated with THC.
To generate gold-standard scientific evidence, bolster its marketing claims and differentiate its product from conventional THC offerings, Phylos partnered with People Science, specialists in technology-enabled decentralized clinical trials. This collaboration gathered concrete scientific evidence for THCV's impact on subjective focus, energy, and activity to position the gummy as a preferred choice in the cannabis market.
Data Collection
The Phylos study targeted healthy people aged 21 and older. The recruitment strategy aimed to evaluate efficacy in the target user-base without imposing strict demographic quotas, resulting in a varied participant pool (71 participants, 61% regular cannabis users, 53% female, 40% Caucasian, 30% Latino, 8% African American, 8% Asian, 14% other ethnicities).
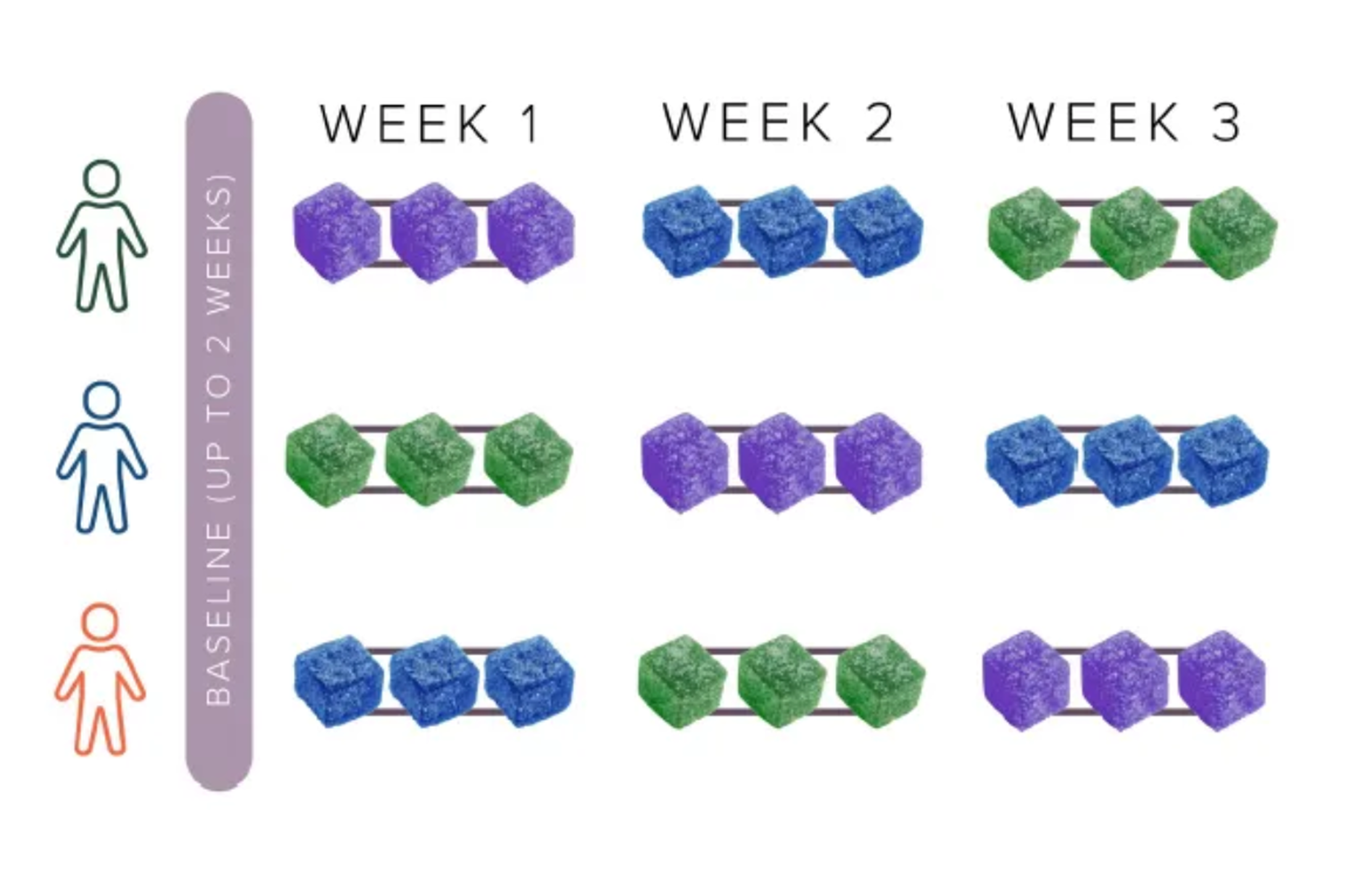
The study spanned 12 weeks, during which participants were asked to consume nine gummies in a randomized order: blocks of three THCV+THC, three THC-only, and three placebo. Participants chose three days per block for consumption and used the Chloe app to log their subjective experiences, including energy levels, focus, exercise performance, appetite, and any side effects. The double-blind design ensured that neither the participants nor the researchers knew which type of gummy was consumed in a given block, enhancing the scientific integrity of the study. Results were published in a poster at ICRS (International Cannabinoid Research Society) in 2024.
Execution
To execute a decentralized, double-blind, placebo-controlled study, People Science utilized its proprietary Consumer Health Learning & Organizing Ecosystem (Chloe). Chloe combines a mobile app with a health research platform designed for companies that prioritize scientific rigor, enabling rapid and cost-effective validation of product claims through evidence-based methods.
Decentralized Clinical Trials (DCTs) evaluate the impact of novel interventions without the need for in-person doctor’s visits. By combining a mobile app for communication, consent, data entry and visualization, a software backend for clinical operations and a team to manage shipments - all the work of participating in a clinical trial can be done from home. This strategy is speeding every stage of scientific advancement, from cost reduction to study design, recruitment, data collection, monitoring, and data visualization.
Study Design
Double-blind, placebo-controlled, double crossover trial (ClinicalTrials.gov ID NCT06213064).
Treatments: placebo, THCV+THC (6.5 mg THCV, 3.4 mg THC), or THC (5 mg THC) gummy; all gummies were red in color and had watermelon flavoring. Participants took each treatment type 3 days in a one-week period, then crossed over into another treatment arm.
Leveraging the Chloe Platform: The Chloe platform played a key role in the Phylos trial, offering a comprehensive solution for decentralized data collection and participant engagement. More than just an app, it simplified every phase of the trial from study design to data capture, reducing logistical challenges for both the trial sponsor and participants.
Chloe’s web platform supports complete trial management, including custom data collection from surveys, wearables, and labs. Fully HIPAA-compliant and FDA audit-ready, the platform streamlines processes such as screening, consent, compliance tracking, and reporting.
Dr. John Doe, PhD, RD
SVP, Science and Innovation at Phylos
Benefits to Phylos
Benefits to
Trial Participants
-
People Science is revolutionizing the landscape of clinical trials by making them more accessible and affordable for consumer health, wellness, and nutrition brands. By integrating advanced technology with clinical expertise, the company redesigns the evaluation process to deliver precise measurement, deeper understanding, and more impactful health outcomes.
-
Real-time feedback on individual progress, making the study experience more engaging.
-
High Data Accuracy is essential for reliable clinical trials, ensuring that every measurement and data point reflects true outcomes without error or bias. At People Science, we leverage advanced technology and rigorous clinical protocols to maximize precision, enabling brands to make informed decisions with confidence.
-
People Science: Revolutionizing Clinical Trials for Consumer Health
People Science transforms the way consumer health, wellness, and nutrition brands conduct clinical trials by merging cutting-edge technology with expert clinical insight. This innovative approach streamlines trial processes, reduces costs, and enhances data accuracy, ultimately delivering more reliable health outcomes. By redefining clinical research, People Science empowers brands to make informed decisions and foster innovation that benefits everyone.
-
People Science is revolutionizing the landscape of clinical trials by making them more accessible and affordable for consumer health, wellness, and nutrition brands. By integrating advanced technology with clinical expertise, the company redesigns the evaluation process to deliver precise measurement, deeper understanding, and more impactful health outcomes.
-
Provided instant insights into participant data, allowing timely adjustments to maximize study effectiveness.
-
High Data Accuracy is essential for reliable clinical trials, ensuring that every measurement and data point reflects true outcomes without error or bias. At People Science, we leverage advanced technology and rigorous clinical protocols to maximize precision, enabling brands to make informed decisions with confidence.
Chloe’s web platform supports complete trial management, including custom data collection from surveys, wearables, and labs. Fully HIPAA-compliant and FDA audit-ready, the platform streamlines processes such as screening, consent, compliance tracking, and reporting.
Susan R.
Study Participant
Other Case Studies

Book a Demo
Turn your customers’ everyday health data into your most powerful competitive asset. Explore Chloe: Consumer Health today.