CASE STUDY
Decentralized Excellence:
People Science and Phylos Bioscience Show that THCV Improves Energy and Wellbeing with Minimal Side Effects
Study Overview
Study facts, key findings, and real-world implications.
Study Snapshot
Primary focus
Evaluate the impact of Phylos’ THCV+THC gummy on energy, focus, and subjective exercise performance vs standard THC and placebo gummies.
Participants
71 participants, 53% female, 40% Caucasian
Study Design
Double-blind, placebo-controlled, randomized, decentralized trial approved by the Advarra Institutional Review Board (IRB).
Study Structure
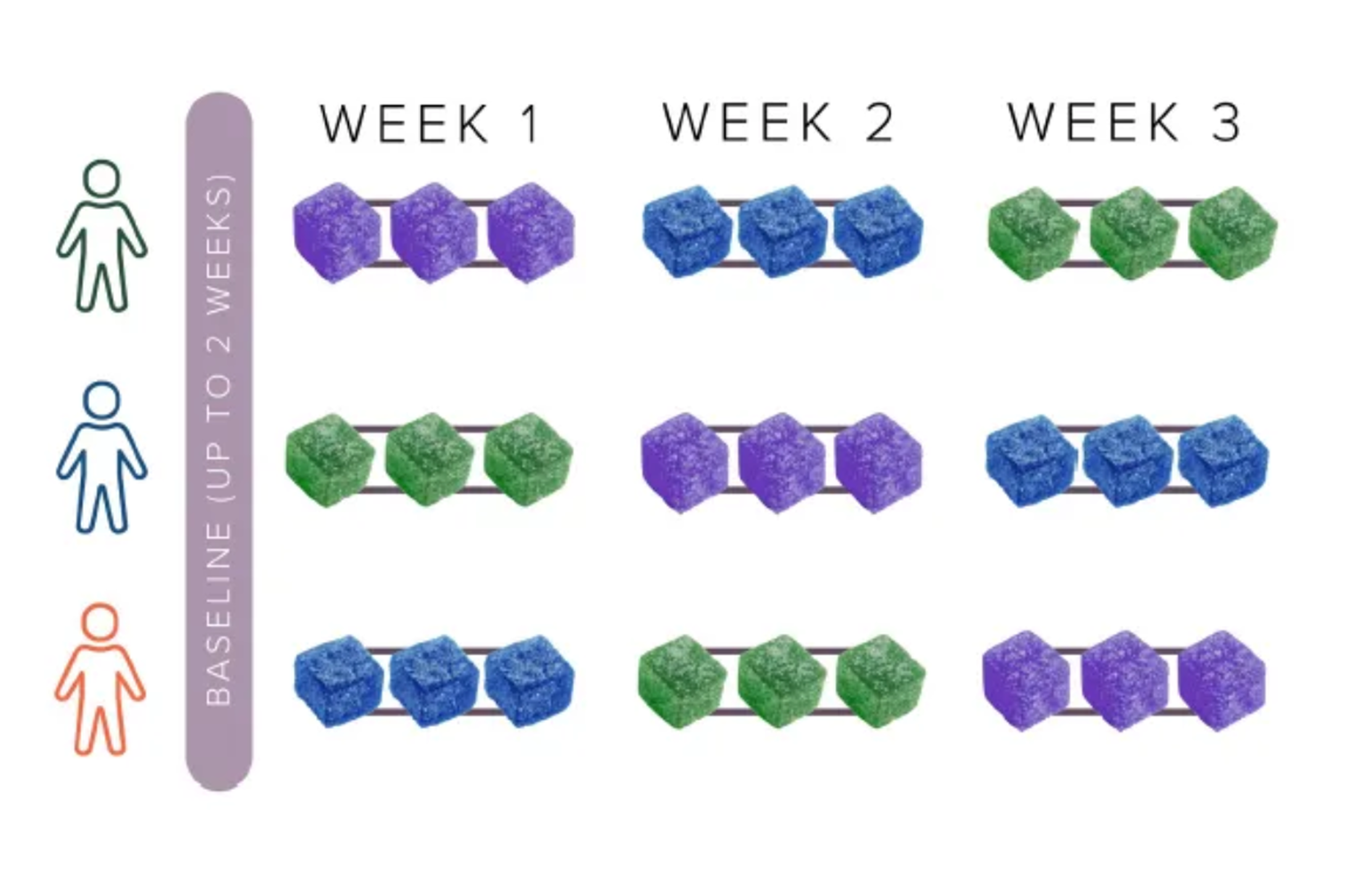
Participants consumed nine gummies (three THCV+THC, three THC-only, three placebos) on a randomized schedule, logging outcomes via the Chloe app.
Duration
2 weeks recruitment, 1 week baseline, 12 weeks intervention
Analytical Methods
General Linear Models to evaluate treatment effects, controlling for individual variation, complemented by non-parametric tests.
Validation
IRB approved. Results published in a poster at ICRS (International Cannabinoid Research Society), 2024.
Key Results
20%
More participants feeling enhanced energy and well-being with THCV+THC gummies.
3x
Fewer fatigue reports for HCV+THC gummies vs THC-only.
0%
Stable appetite for THCV+THC gummy, vs 50% rise in hunger for THC-only gummies.
40%
more participants enjoyed their daily activities after taking the THCV+THC gummy compared to the placebo.
98%
Participant reported positive trial experience and willing to join future studies.
Study Details
Introduction
Phylos Bioscience, a leader in cannabis genetics, embarked on an innovative journey to validate the efficacy of its THCV+THC gummy—a product crafted to enhance energy, sharpen focus, and improve subjective athletic performance while minimizing the fatigue and hunger associated with THC.
Tetrahydrocannabivarin (THCV), a non-psychoactive variant of tetrahydrocannabinol (THC), presents a promising wellness alternative to THC-only products and traditional medicines. However, clinical evidence is limited, as are descriptions of real-world differences from impacts of THCV.
To bolster its marketing claims and differentiate its product from conventional THC offerings, Phylos partnered with People Science, specialists in technology-enabled decentralized clinical trials. This collaboration gathered concrete scientific evidence for THCV’s impact on subjective focus, energy, and activity to position the gummy as a preferred choice in the cannabis market.
Data Collection
The Phylos study targeted healthy people aged 21 and older. The recruitment strategy aimed to evaluate efficacy in the target user-base without imposing strict demographic quotas, resulting in a varied participant pool (71 participants, 61% regular cannabis users, 53% female, 40% Caucasian, 30% Latino, 8% African American, 8% Asian, 14% other ethnicities).
The study spanned 12 weeks, during which participants were asked to consume nine gummies in a randomized order: blocks of three THCV+THC, three THC-only, and three placebo. Participants chose three days per block for consumption and used the Chloe app to log their subjective experiences, including energy levels, focus, exercise performance, appetite, and any side effects. The double-blind design ensured that neither the participants nor the researchers knew which type of gummy was consumed in a given block, enhancing the scientific integrity of the study. Results were published in a poster at ICRS (International Cannabinoid Research Society) in 2024.
Execution
To execute a decentralized, double-blind, placebo-controlled study, People Science utilized its proprietary Consumer Health Learning & Organizing Ecosystem (Chloe). Chloe combines a mobile app with a health research platform designed for companies that prioritize scientific rigor, enabling rapid and cost-effective validation of product claims through evidence-based methods.
Decentralized Clinical Trials (DCTs) evaluate the impact of novel interventions without the need for in-person doctor’s visits. By combining a mobile app for communication, consent, data entry and visualization, a software backend for clinical operations and a team to manage shipments - all the work of participating in a clinical trial can be done from home. This strategy is speeding every stage of scientific advancement, from cost reduction to study design, recruitment, data collection, monitoring, and data visualization.
Study Design
Double-blind, placebo-controlled, double crossover trial (ClinicalTrials.gov ID NCT06213064).
Treatments: placebo, THCV+THC (6.5 mg THCV, 3.4 mg THC), or THC (5 mg THC) gummy; all gummies were red in color and had watermelon flavoring. Participants took each treatment type 3 days in a one-week period, then crossed over into another treatment arm.
Leveraging the Chloe Platform: The Chloe platform played a key role in the Phylos trial, offering a comprehensive solution for decentralized data collection and participant engagement. More than just an app, it simplified every phase of the trial from study design to data capture, reducing logistical challenges for both the trial sponsor and participants.
“This study signifies a pivotal moment for the cannabis industry in understanding the effect of THCV in combination with THC. The study allowed us to leverage our exceptional plants in the advancement of targeted cannabis products, and to chart new territories in understanding the efficacy of natural cannabinoids.”
Alisha Holloway, PhD
Chief Scientific Officer at Phylos
Benefits to Phylos
-
The trial bolstered Phylos’ market credibility by providing scientific validation for the benefits of the THCV+THC gummy, enhancing consumer trust and brand reputation.
The scientific findings from the trial provided Phylos with the validation needed to launch a new business line, Natural Natural. This consumer-facing ingredient brand commercializes Phylos’ patented, naturally-derived rare cannabinoid genetics, offering science-backed, plant-based wellness products. Leveraging its proven effects, Natural Natural has partnered with leading growers, extractors, and brands to ensure the highest quality standards, expanding its reach across North America and setting the stage for further growth.
Findings garnered media attention, were presented at scientific conferences, and are in preparation for peer-reviewed publication, positioning Phylos as an industry innovator. Following the trial’s success, Phylos plans to expand research and explore additional product lines.
-
Enabled seamless remote participation, reducing logistical barriers and costs associated with in-person visits.
-
Provided instant insights into participant data, allowing timely adjustments to maximize study effectiveness.
-
Structured prompts and reminders helped maintain high data quality and participant adherence throughout the trial.
Benefits to
Trial Participants
-
Allowed participants to track their experiences with the THCV+THC gummies from home, enhancing accessibility.
-
Real-time feedback on individual progress, making the study experience more engaging.
-
Provided personal and group-wide data reports after the study, empowering participants with knowledge of the outcomes.
-
The platform’s usability scored 4.5/5, with 98% of participants satisfied, willing to join future studies, and likely to recommend Chloe to their peers.
“This was a delightful experience. Thank you for showing me an alternative to consuming edibles that help you tremendously. Especially when the help comes from studies.”
Susan R.
Study Participant
Other Case Studies

Book a Demo
Turn your customers’ everyday health data into your most powerful competitive asset. Explore Chloe: Consumer Health today.