CASE STUDY
Decentralized Excellence:
People Science and Verb Biotics published clinical evidence that probiotic LP815 significantly improved symptoms of anxiety compared to a placebo.
Study Overview
A look at the study facts, key findings, and real-world implications.
Study Snapshot
Primary focus
Clinically evaluate the impact of two doses of LP815 probiotic versus a placebo on anxiety. Drive product development, support claims and generate peer-reviewed published research.
Participants
83 participants, 63% female, 64% Caucasian.
Study Type
Double-blind, placebo-controlled, randomized, decentralized trial approved by the Advarra Institutional Review Board (IRB).
Study Structure
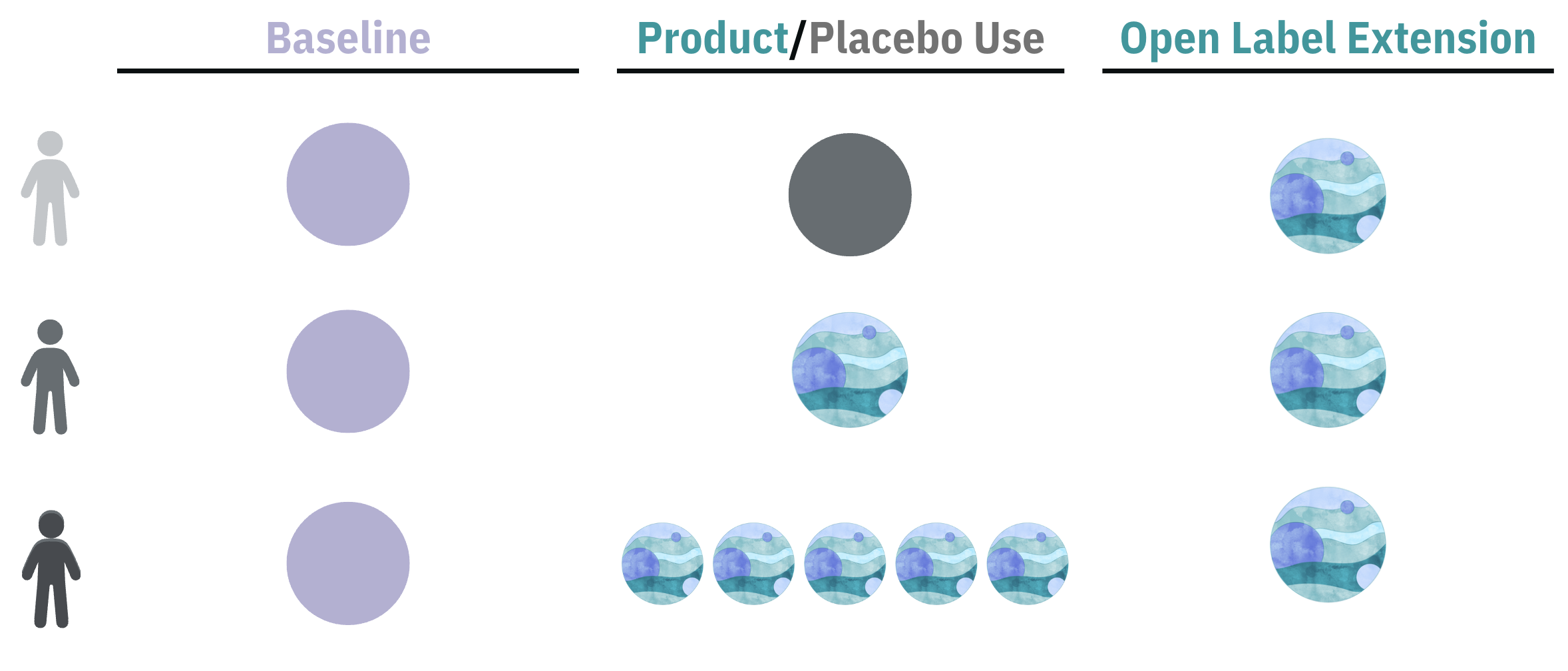
Participants collected one week of baseline data followed by 6 weeks of product or placebo use. Participants could opt into an Open Label Extension. This study was completely decentralized. Outcomes were collected in Chloe mobile app.
Duration
3 weeks recruitment, 1 week baseline, 6 weeks intervention
Analytical Methods
Non-parametric statistics (Holm’s and Dunn’s corrected Kruskal-Wallis) and Mann-Kendall trend over time tests.
Validation
IRB approved. Published in Beneficial Microbes.
Key Results
151%
Anxiety improvement, 68% of participants’ anxiety scores improved 1+ category.
p=0.04
Reduced Feelings of Irritability and Annoyance.
ISI
Trend Toward Reducing Sleep Disturbance Symptoms.
96%
Participants very satisfied, study experience average rating = 9.3/10.
99%
Completion
Study Details
Introduction
Verb Biotics, a leader in the development of effective and novel probiotic strains, tested the effectiveness of lactiplantibacillus plantarum 815 (LP815) — a strain selected in the laboratory to produce the inhibitory neurotransmitter GABA in the physiological pH range. Our trial sought to determine if LP815 could impact symptoms of anxiety, sleep disturbance and cognition using the gold-standard research design: a randomized, placebo-controlled trial in a real-world setting.
The major inhibitory neurotransmitter gamma-aminobutyric acid or GABA plays a pivotal role in mood and sleep. GABA exerts sedative and anxiolytic effects both within the central nervous system and through the gut-brain axis, which has generated interest in the potential for gut GABA to modulate mood and sleep. The present study is the largest to date investigating the impacts of a GABA-producing probiotic.
To bolster its marketing claims and differentiate LP815 from conventional probiotics, Verb Biotics partnered with People Science, specialists in technology-enabled decentralized clinical trials. This collaboration gathered concrete scientific evidence for LP815's impact on symptoms of anxiety, poor sleep and cognition.
Data Collection
Participants collected 1 Week (W) of baseline data, using the Chloe app to log their demographics and medical history, anxiety symptoms (validated GAD-7), sleep disturbance symptoms (validated ISI), wearable data and cognition. This was followed by 6 weeks of product or placebo use. The double-blind design ensured that neither the participants nor the researchers knew which group took the products or placebo, enhancing the scientific integrity of the study. After 6 weeks, all groups had the opportunity to try the 1 Billion CFU probiotic as part of an Open-Label Extension, ensuring all participants had the chance to test the active product..
Execution
Chloe streamlined study design, recruitment, data collection, monitoring and data visualization, providing a comprehensive approach to decentralized clinical trials.
Recruitment targeted healthy adults with mild-to-moderate symptoms of anxiety. Recruitment leveraged community connections and targeted advertising to ensure diversity without imposing strict demographic quotas, resulting in a varied participant pool (83 participants, 63% female, 64% Caucasian).
The study spanned 7 weeks, including 1 week of baseline and 6 weeks of daily low dose probiotic (1 Billion CFU), high dose probiotic (5 Billion CFU) or placebo. Intervention adherence rate was 95%.
Study Outcome
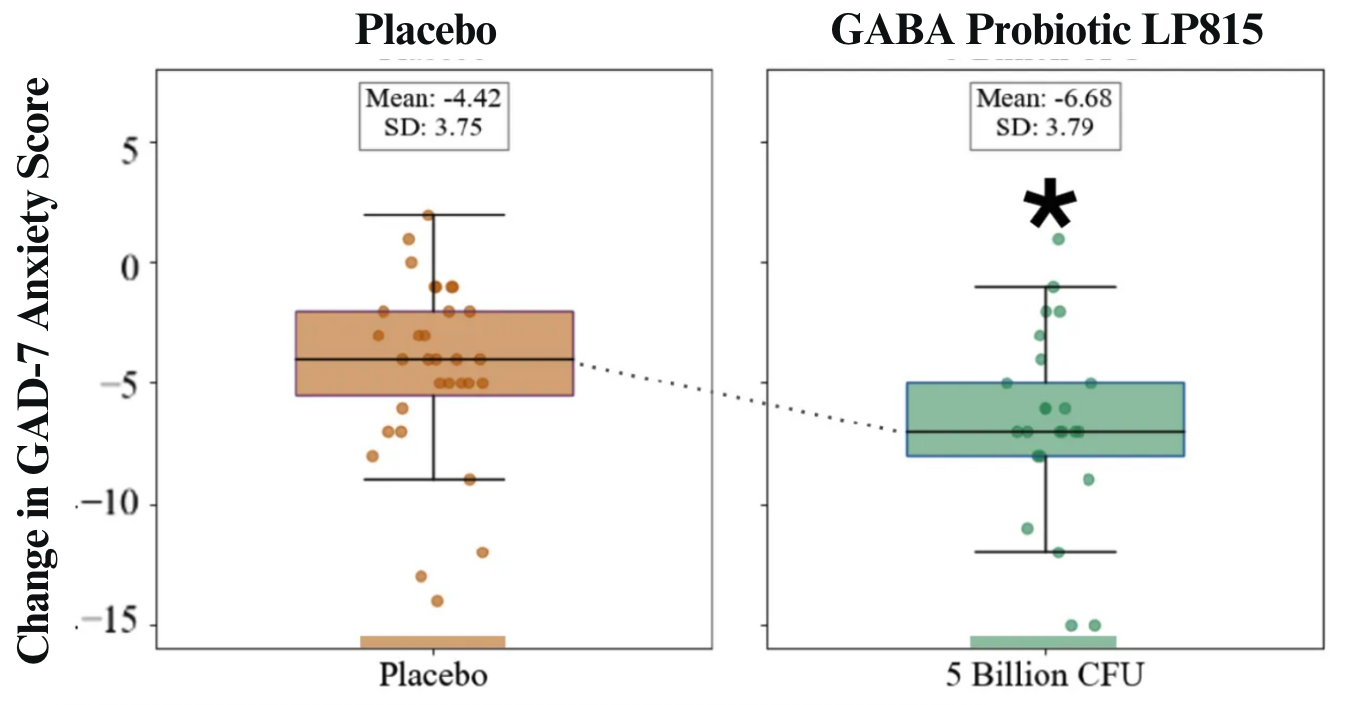
The collaboration yielded statistically significant, publishable results. The study found that GABA Probiotic LP815TM reduced anxiety symptoms, feelings of irritability and annoyance and trended toward reducing symptoms of sleep disturbance. Caption: Box and whisker plots of change from baseline in validated GAD-7 anxiety score after 6 weeks of Placebo (left, orange) or 5 Billion CFU daily LP815TM (right, green). Dots represent individual participants’ data. Those taking LP815TM dropped their anxiety by 6.68 points, compared to 4.42 in placebo. That’s 151% greater improvement.
We completed recruitment in just 3 weeks with study assessments completed over 7 weeks. The project demonstrated People Science’s full-service support capabilities, from study design to data analysis and publication management, leveraging the Chloe platform to simplified every phase of the trial from study design to data capture, reducing logistical challenges for both the trial sponsor and participants.
“Our trial with People Science was a clear success and allowed us to make the key business decision to pursue both anxiety and now potentially the sleep market. They offered essential guidance on selection of accurate metrics, executed rapid under-budget recruitment and data analysis and prepared our manuscript within a couple of weeks. The experience and critical thinking of the scientists and staff were invaluable for this trial. We’ll be sticking with PS for our subsequent trial and would highly recommend them to our colleagues.”
Noah Zimmerman, PhD
CTO at Verb Biotics
Benefits to Verb Biotics
Benefits to
Trial Participants
-
Allowed participants to track their experiences with the probiotic from home or on-the-go, enhancing accessibility. All participants had the opportunity to try the probiotic at end of study via an Open-Label Extension.
-
All participants received personalized in-app results at the end of the study, as well as first access to the overall study results.
-
The platform’s usability scored 4.5/5, with an average rating of trial experience of 9.3/10. 96% of participants were very satisfied, willing to join future studies and likely to recommend Chloe to their peers.
-
This RCT provided gold-standard evidence that Verb’s GABA Probiotic product improves anxiety, strengthening brand reputation and setting the ingredient apart from competitors.
Findings were published in a respected journal focused on the microbiome and probiotics, adding credibility to Verb’s claims. Peer-reviewed publication enabled Verb to attract media attention and present its findings to the scientific and industry community. Critically, trials like these help validate product claims for Verb’s customers, strengthening trust and confidence in the supplier relationship. This foundation of evidence positions Verb as a preferred partner and “go-to” supplier. Following this trial, Verb expanded its research to explore additional benefits of its products and their mechanistic underpinnings.
-
Enabled seamless remote participation, reducing logistical barriers and costs associated with in-person visits.
-
Provided instant insights into participant data, allowing timely adjustments to maximize study effectiveness.
-
Structured prompts and reminders helped maintain high data quality and participant adherence throughout the trial.
“This app made this whole process super smooth and manageable.”
“I’m loving this study.”
2 Study Participants
Other Case Studies

Book a Demo
Turn your customers’ everyday health data into your most powerful competitive asset. Explore Chloe: Consumer Health today.